Abstract
Introduction: Optimal initial therapy for mantle cell lymphoma (MCL) remains uncertain. Bendamustine-rituximab (BR) is commonly used. Rituximab consolidation is often added post-BR but lacks randomized supporting data. Bortezomib (V) and lenalidomide (L) have activity against MCL. The randomized phase 2 NCTN trial E1411 tested if progression-free survival (PFS) is prolonged by addition of bortezomib (V) (1.6 mg/m2 SC/IV days 1, 8) to BR (BVR vs BR) induction and/or by addition of lenalidomide (L) (15 mg/d days 1-21/28) to rituximab (LR vs R) consolidation. Prior report (ASCO 2021) indicated no significant benefit of addition of V to BR. Here we report efficacy and toxicity of LR vs R consolidation.
Methods: E1411 randomized 373 patients into induction (step 1) between 2012-16, stratified by MIPI (low v intermediate v high) and age (<60 v ≥60). Randomization was to 1 of 4 arms: A) BR induction x 6 followed by R x 2 yrs, B) BVR followed by R, C) BR followed by LR, or D) BVR followed by LR. Eligible pts had untreated MCL, ≥ age 18 (amended from ≥60 when S1106 for < 65 closed), ECOG PS 0-2 and adequate hematologic and organ function. Pts without progressive disease (PD) during induction proceeded to consolidation (step 2) R vs LR. For the endpoint of PFS from start of consolidation, patients were stratified by induction strategy (BR vs BVR) for the pooled analysis of LR vs R. For step 2, 290 eligible treated consolidation patients were required to provide 89.4% statistical power to demonstrate a 37.4% reduction in the hazard ratio, using stratified log-rank test with a 10% 1-sided alpha.
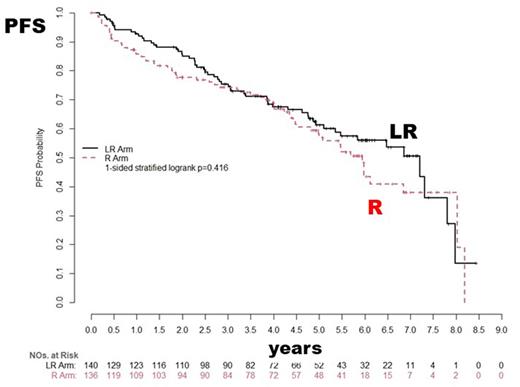
Results: Demographics did not differ between the groups: median age 67 (range 42-90), 73% men, ECOG PS 0-1 98%, MIPI Low/Intermed/Hi 40/31/29%. Of 293 patients that registered to consolidation, the efficacy population was 276 (17 ineligible or not treated). The planned number of consolidation treatment cycles were completed in 95/140 (67.9%) LR vs 90/136 (66.2%) R; off treatment reasons were PD 11 vs 22, AEs 15 vs 7, other 19 vs 17, respectively. Estimated PFS at 2-yrs 85.8% LR (95% CI 78.6-90.7) vs 77.7% R (95% CI 69.5-84.0) (1-sided stratified log-rank p=0.416); hazard ratio for LR vs R = 0.96 (80% CI 0.75, 1.23). Of 119 PFS events (57 LR; 62 R), 29 are death without PD, 40 with PD have died, 50 alive post-PD. With median PFS follow-up of 5.8 years, median PFS estimated in the pooled arms is 6.1 years from step 2 registration. Of 74 pts not in CR post-induction, 50% (n=37) converted to CR (18 on LR & 19 on R arm).
Calculating overall PFS from study registration (includes induction + consolidation), the 2-yr PFS and median PFS (years) for the 4 individual arms were BR-R (80.7%, 6.0), BVR-R (82.8%, 6.6), BR-LR (83.3%, 7.8), BVR-LR (94.2%, 7.7). Limiting the analysis to patients over age 60 (87% of patients on study) the 2 year and median PFS in each arm, starting from study registration, was BR-R (81.2%, 6.0), BVR-R (81.0%, 6.2), BR-LR (81.2%, 5.7), BVR-LR (93.2%, 7.7).
Grade ≥ 3 toxicities were 87.0% LR vs 64.1% R, mostly neutropenia 58.9 vs 19.7%, with febrile neutropenia 6.8 vs 3.5%. The only non-hematologic grade ≥ 3 toxicity in > 5% of pts was lung infection/pneumonitis/URI (9.6 vs 4.2%); with 3 potentially treatment-related deaths (MI & invasive SCC on LR, MDS on R). Second malignancies by arm were: BR-R (17), BVR-R (21), BR-LR (23), BVR-LR (34) [data is from 64 unique patients; 19 patients had >1 second malignancy].
Conclusions: Lenalidomide did not significantly improve the primary endpoint of PFS when added to R as consolidation of BR-based induction as initial MCL therapy. Further follow-up continues as > ½ of pts remain alive and without progression. Each treatment arm was tolerable and deliverable to the majority of patients. The high CR rate and mPFS of 6+ years from start of BR-based induction + R-based fixed duration consolidation support this approach for 1st-line MCL therapy in individuals not appropriate for more intensive approaches, and as a platform for broader clinical trials (e.g. EA4181).
Disclosures
Smith:jannsen: Honoraria; acrotech: Speakers Bureau. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Till:Mustang Bio: Consultancy, Patents & Royalties, Research Funding; Proteios Technology: Consultancy; BMS/Celgene: Research Funding. Casulo:Genentech: Research Funding; Bristol Myers Squibb: Research Funding; Gilead: Research Funding; Secura Bio: Research Funding; Verastem: Research Funding. Caimi:Novartis: Consultancy; Kite: Consultancy; Genentech: Consultancy; MEI Pharma: Honoraria; GenMab: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Research Funding; Incyte: Consultancy; Janssen: Consultancy; BMS: Honoraria. Al Baghdadi:BMS: Current equity holder in publicly-traded company, Honoraria; Heron Therapeutics: Current holder of stock options in a privately-held company; Cardinal Health: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Eli Lilly: Membership on an entity's Board of Directors or advisory committees. Maddocks:Janssen: Consultancy; Genentech: Consultancy; BMS: Consultancy, Research Funding; Abbvie: Consultancy; ADC Therapeutics: Consultancy; Pfizer: Research Funding; Pharmacyclics: Consultancy, Research Funding; Incyte: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Acerta: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Genmab: Consultancy; Kite: Consultancy; Lilly: Consultancy; Morphosys: Consultancy. Wagner:Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Leonard:Second Genome: Consultancy; Seattle Genetics: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Mustang Bio: Consultancy; Merck: Consultancy; MEI Pharma: Consultancy; Lilly: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Grail: Consultancy; Gilead/Kite: Consultancy; Genmab: Consultancy; Roche/Genentech: Consultancy; Epizyme: Consultancy; Eisai: Consultancy; Constellation: Consultancy; Calithera: Consultancy; Bristol-Myers Squibb: Consultancy; Beigene: Consultancy; Bayer: Consultancy; AstraZeneca: Consultancy; Astellas: Consultancy; AbbVie: Consultancy; Sutro: Consultancy; ADC Therapeutics: Consultancy; BMS/Celgene: Consultancy; Regeneron: Consultancy; Miltenyi: Consultancy. Kahl:Janssen: Consultancy; Kite: Consultancy; Beigene: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy; AcertaPharma: Consultancy; MEI: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Roche: Consultancy; ADT Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Incyte: Consultancy; Hutchmed: Consultancy, Research Funding; TG Therapeutics: Consultancy; Genmab: Consultancy; Seattle Genetics: Consultancy; Research To Practice: Speakers Bureau.
OffLabel Disclosure:
lenalidomide as maintenance after BR induction rituximab as maintenance after BR induction
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal